Degradation Influencing Factors of Electrolysis System in Dy
| 论文类型 | 技术与工程 | 发表日期 | 2005-11-01 |
| 作者 | Zhemin,Shen,Wenhua. | ||
| 摘要 | Degradation Influencing Factors of Electrolysis System in Dye Treatment and Application of Nanophase Catalyst Zhemin Shen , Wenhua. Wang, Yi Xu, Yanming Wang, Hai Yan, Hongxiao Tang State Key Laborat | ||
Degradation Influencing Factors of Electrolysis System in Dye Treatment and Application of Nanophase Catalyst
Zhemin Shen , Wenhua. Wang, Yi Xu, Yanming Wang, Hai Yan,
Hongxiao Tang
State Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco-environmental Sciences, Chinese Academy of Sciences, Beijing, 10085, P. R. China.
1. Introduction
Effluents from textile and dye synthesizing industry usually contain dyes. Although dyes make a small contribution to the typical parameters used to measure pollutant concentrations, there is increasing concern about them due to their resistance to degradation in typical biological wastewater treatment systems where almost no color removal is achieved. Also its high pH, temperature and large amount of suspended solids are well known (S. DeFazio et al, 1999; A. G.Vlyssides et al 1998;).
Many methods have been conducted with respect to decolorization of these kinds of wastewater. Because of its large variability of the composition, most traditional methods are becoming inadequate. Chlorination and ozonation have been proposed as potential alternatives. However, the cost or the secondary toxic byproducts arising from the residual chlorine needs to be further ascertained to insure the competence of them. Activated carbon adsorption is another efficient decoloration method. It is not desirable because of high running and raw costs (S. Lin et al 1996; A. G.Vlyssides et al 1999.).
Electrochemical technology has been paid increasingly more attention to, because of its success in color removal (S. DeFazio et al, 1999; A. G.Vlyssides et al 1998; S. Lin et al 1996.). Advances in electrode materials have led to progress in electrolytic effectiveness (J. Rodgers et al, 1999; E. Brillas et al, 1998.). In the past graphite has been frequently used as an anode during electrochemical treatment as it is relatively economical and gives satisfactory results. Titanium electrodes covered with very thin layers of electrodeposited noble metals have recently been used for electro-oxidation. Electrodes can also be coated with ruthenium, rhodium, lead and stannum oxide (A.G. Vlyssides et al, 1998). These high oxygen overvoltage anodes have been used in destroying pollutants in wastewater (M. Nicola, et al, 1996; M.Tezuka et al, 1996; J.Casado et al, 1996.). N. Taira and his coworkers (T. Naohide et al, 1998) have treated dyestuff using PbO2 anode. In their study, Orange II was decolorized completely by a 120 min electrolysis using PbO2 anode at current density of 0.2 A cm-2. ; A.M. Polcaro (A.M. Polcaro et al 1999) has also studied the performance of Ti/PbO2 anode during electrolysis of 2-cholorophenol in terms of faradaic yield and fraction of toxic intermediates removed. Graphite and Ti/PbO2 anodes were used as anodes in this study.
However, the existence of catalyst in the electric field can also enhance the treatment efficiency. W. Chen (W. Chen et al,1998) have used metal dioxide to treat 3 types of organic pollutants, phenol, phenylamine and di-Me phthalate from wastewater. The existence of H2O2 and ·OH were verified during electrolytic catalysis process. The results showed that removal efficiency depended on the lived intermediate yield in the system. Organic pollutants were effectively removed when H2O2 yield increased. Notable removal efficiency reached when H2O2 yield was more than 0.3 mg/L. Semiconductor catalyst can be used in electrochemical process as well as in photo-degradation system [A. Taghizadeh et al, 2000; E. Moctezuma et al 1999]. The removal of xenobiotic compounds, such as chlorophenols and pesticides, from municipal and industrial wastewater is an important task because of the toxicity and the tendency to bioaccumulation of these compounds. Among the several methods proposed, photo-degradation catalyzed by suspended inorganic semiconductors (i.e. TiO2) has lately received wide attention because this process leads to non-toxic final products and shows a high degradation efficiency ( L. Campanella et al, 1995.). TiO2 is a semiconductor catalyst that has been widely studied since the seventies. TiO2 electrode has used degrading 4 chlorophenol and dyes (J.M. Kesselman, 1997, R. Pelegrini et al, 1999 ). Nanophase TiO2 Catalyst is studied in this research.
Acid dyes are the popular choice of dying nylon or polyamide fiber, wool and silk. This wok studied the treatment of Acid Red B (Acid Red 14, an azo dye). Its structural formula is as following.
2. Materials and Methods
Color was analyzed by U-3010 Spectrophotometer (Hitachi, Japan). Each dye solution was scanned and its maximum absorbency visible wavelength was detected.
Dye concentration was 60mg L-1. Solution contained Na2SO4 as an electrolyte. Electric current density was detected with the time.
3 Results and Discussions
3.1 Electrolyte Concentration
As shown in Fig.1, the higher the electrolyte concentration is, the larger the conductance and the electric current of the solution. The figure of conductance is almost a straight line. Electrolyte enhances the conductance of the solution. While, the figure of electric current is a little winding. This lies in the alteration of potential allocation between electrodes and in solution.It is made clear in Fig.2 that with salt concentration are enhanced, solution resistance decreases and potential difference in solution decreases. However, potential difference on electrodes to overcome interface resistance increases.
Electrolysis results in various electrolyte concentrations at 8V, 40min is shown in Fig.3. More salt can improve the color removal effect. However, Color removal is not proportional to the electric current and salt concentration. At low salt concentration, color removal improves quickly with more salt added in the solution. At high salt concentration, it increases slightly. As potential difference on electrode interface is more direct to the degradation process, higher electrode interface potential difference leads to deeper degradation. So the color removal curve is a little winding like the electrode interface potential difference curve.
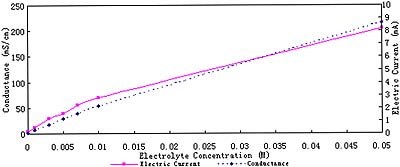
Fig. 1 Conductance and electric current (at 8V) of various electrolyte concentration
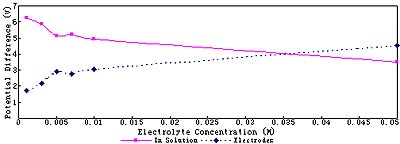
Fig.2 Potential allocation (at 8V) of various electrolyte concentration
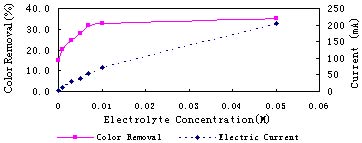
Fig.3 Electrolysis results in various electrolyte concentration at 8V, 40min
3.2 Temperature
Electrolysis results at various temperatures are shown in Fig.4. High temperature increases the activity of solution molecules. Consequently, conductance and electric current increase with the enhancement of temperature. So is the color removal effect.
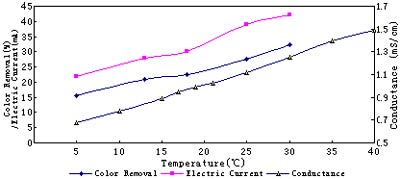
Fig. 4 Electrolysis results at various temperatures at 8V, 40min
3.3 Stirring and Voltage
Two stirring methods, magnetic stirring and aerating have been tested. Electrolysis results under different stirring conditions are given in Fig.5. It is clear that aerating and magnetic stirring can improve degradation effect, for they can weaken polarization effect in electrolysis process. Also, it is indicated in Fig. 5 that great electric voltage can improve the degradation effect.
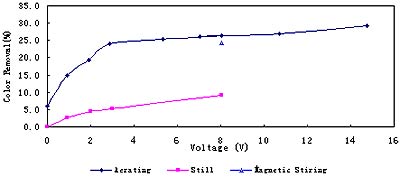
Fig. 5 Electrolysis results under different stirring conditions and at various voltages
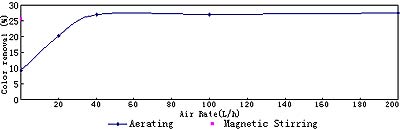
Fig. 6 Electrolysis results at various air rates at 8V, 40min
The results at various air rates are shown in Fig.6. Of course, high air rate and drastic stirring lead to small polarization and great degradation. However, after air rate reach certain value, about 40 L/h, its promotion effect is slight.
As for these three kinds of influencing factors, electrolyte, temperature and electric voltage, their effects are compared in Fig.7. All of them can improve electric current degradation effect. However, their utilization efficiencies of electric current are different. Slope rate of temperature is the biggest among three curves as a whole. So its current usage is the highest. As for voltage curve, at low voltages its increase greatly enhances the degradation process. After voltage reach about three volts, its usage declines quickly. At high voltages most of current is consumed by side reactions.
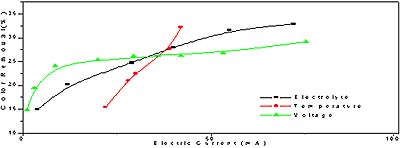
Fig.7 Comparison of three kinds of influencing effect
3.4 Nanophase Catalyst
Fig.8 shows electrolysis results of TiO2 nanophase catalyst Mixed with 5% (mol/mol) other elements. Evidently, Co, Zn and W can enhance dye removal.
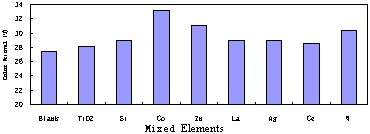
Fig.8 Electrolysis results of TiO2 nanophase catalyst Mixed with 5%(mol/mol) other elements
In order to select more efficient catalysts, orthogonal experiments are designed. Four factors and four levels are selected to test. Tab.1 gives the results of orthogonal experiments. It is indicated that temperature is more influential to catalysis effect than other factors. Bi has smallest effect on the catalyst results. And catalyst has best affectivity prepared at 400 and mixed with 10% Co.
NO. Temperature (°C) Co (mol/mol) W (mol/mol) Bi (mol/mol) Color removal (%) Color removal (%) Average (%) Standard Deviation 1 400 0% 0% 20% 33.5 34.1 33.8 0.424 2 400 5% 5% 5% 34.4 36.7 35.55 1.626 3 400 10% 20% 2% 41.3 44.1 42.7 1.980 4 400 20% 50% 0% 42.2 31.4 36.8 7.637 5 450 0 0.05 0.02 29.5 30.6 30.05 0.778 6 450 5% 0% 0% 43.9 33.5 38.7 7.354 7 450 10% 50% 20% 37.6 33.5 35.55 2.899 8 450 20% 20% 5% 33.4 36.6 35 2.263 9 550 0 0.2 0 29.5 33.1 31.3 2.546 10 550 5% 50% 2% 27.1 27.8 27.45 0.495 11 550 10% 0% 5% 37.5 30.9 34.2 4.667 12 550 20% 5% 20% 27.6 30.9 29.25 2.333 13 750 0 0.5 0.05 34.5 29.9 32.2 3.253 14 750 5% 20% 20% 31.4 32.9 32.15 1.061 15 750 10% 0% 0% 32.9 29.5 31.2 2.404 16 750 20% 5% 2% 36.2 31.3 33.75 3.465 K1 148.85 127.35 137.9 130.75 539.65 45.184 K2 139.3 133.85 128.6 136.95 K3 122.2 143.65 141.15 133.95 K4 129.3 134.8 132 138 Sum 18303 18235.1 18225.4 18209.4 18201.4 Sum of squares of deviations 101.65 33.67 24.04 7.98 213.25
To confirm the result of the orthogonal experiments, three nanophase composite catalysts have been prepared at 400°C. Their catalysis effect is shown in Fig.9. Catalyst mixed with10% (mol/mol) Co and 20% W (mol/mol) is less efficient than mixed with 10% 。Its decorization effect increases about four percent and is about 1.15 times of the color removal without catalyst.
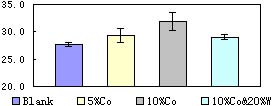
Fig.9 Catalysis effect of three kinds of nanophase composite catalyst prepared at 400°C
Fig.10 shows a typical X ray diffraction of nanophase composite TiO2 catalyst prepared at 400°C. TiO2 prepared at this temperature is made of anatase structure. Its crystal diameters have been calculated and the results are shown in Tab.3. Its average diameter is less than 20 nonometer.
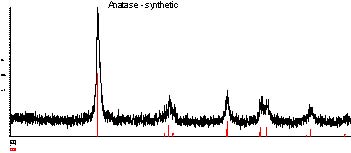
Fig.10 Typical X ray diffraction of nanophase composite TiO2 catalyst prepared at 400°C
Tab.3 Crystal Diameters of nanophase composite TiO2 catalyst prepared at 400°C
4 Conclusions
The result confirms that though these three kinds of influencing factors, electrolyte, temperature and electric voltage, all of them can improve electric current and degradation effect, their utilization efficiencies of electric current are different. Enhancement of temperature to increase electric current is the best way to improve degradation effect, among the three methods. Its current usage is the highest. As for enhancement of voltage, at low voltages its increase greatly enhances the degradation process. After voltage reach about three volts, its usage declines quickly. At high voltages most of current is consumed by side reactions.
Nanophase composite TiO2 catalyst has been prepared to expedite color removal. It is shown that TiO2 prepared at 400℃ is made of anatase structure. Nanophase composite TiO2 catalyst prepared at 400℃ and mixed with 10% Co is effective in this degradation process.Its decorization effect increases about four percent and is about 1.15 times of the color removal without catalyst However, this catalytic method does not increase electric current. Its application potential will be greater if more effective catalyst is invented.
close
www.h2o-china.com论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 [email protected] ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








