Help small systems remove THMs
| 论文类型 | 基础研究 | 发表日期 | 2005-10-01 |
| 作者 | 佚名 | ||
| 摘要 | Help small systems remove THMs Chlorination of drinking water has all but eliminated waterborne diseases such as typhoid fever, cholera and dysentery. Proper chlorination kills the bacteria, viruses and parasites responsible for these il | ||
Help small systems remove THMs
Chlorination of drinking water has all but eliminated waterborne diseases such as typhoid fever, cholera and dysentery. Proper chlorination kills the bacteria, viruses and parasites responsible for these illnesses.
Unfortunately, there are several byproducts from the chlorination of drinking water that pose a possible health risk. Some of these byproducts include trihalomethanes (THMs), haloacetic acids, bromate and chlorite.
Chloroform, bromoform, dibromochloromethane, and dichlorobromomethane make up what are known as the trihalomethanes, which are created when the disinfectant (chlorine) used in water treatment reacts with bromide or natural organic matter (decaying vegetation) present in the source water.
A US Environmental Protection Agency (EPA) survey discovered that THMs are present in virtually all chlorinated water supplies. For many years now, the EPA required large water systems to reduce total THM (TTHM) levels in potable water. Recently, the EPA has required smaller water systems to reduce TTHM levels as well.
While it is true that compared to the waterborne pathogens, the health risks associated with TTHMs are small, these risks are among the most important water quality issues to be addressed in US water supplies.
Examining the THM issue
The TTHM health issues are far reaching, with possible links to heart, lung, kidney, liver, central nervous system damage and bladder/colorectal cancer. A California study found a miscarriage rate of 15.7 percent for women who drank five or more glasses of cold water containing more than 75 parts per billion (ppb) TTHM, compared to a miscarriage rate of 9.5 percent for women with low TTHM exposure. For these reasons, TTHM levels in public water supplies are limited to 0.08 parts per million (ppm) (80 ppb).
There are many options, including reduction of chlorine use at the treatment plant and switching to alternative methods of disinfection, such as chloramines, ozone and ultraviolet (UV), for the reduction of TTHMs.
Chloramine disinfection does reduce TTHM levels because chloramines do not react with and consume the natural organics in the water. This can cause color problems in drinking water since color comes from the tannins and humics derived from decaying plant matter present in the water.
Also, chloraminated water cannot be used for aquariums and kidney dialysis. Activated carbon can be used to remove the color and residual chloramines from the water, but it can also be used to remove the TTHMs from the chlorinated drinking water in the first place.
Activated carbon can be used to remove TTHMs from chlorinated water. The following will discuss carbon characteristics that are desired to optimize the carbon抯 performance for TTHM removal.
Understanding the selection process
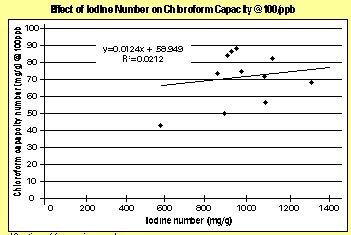
Selection of the correct activated carbon is key to the success of applying activated carbon to any application, and TTHMs are no exception. Traditionally, the parameter used when selecting a carbon is Iodine Number, which is not very effective at gauging a carbon performance at adsorbing trace levels of contaminants. As shown in the figure 1, no correlation exists between Iodine Number and chloroform capacity for a representative sampling of numerous activated carbons.
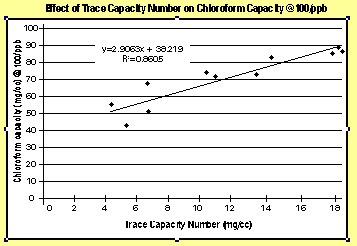
Given the limitations of Iodine Number in trace removal applications, carbon manufacturers are beginning to use new measures to better characterize an activated carbon trace capacity. One new measure, called the Trace Capacity Number, measures a carbon capacity to adsorb acetoxime, a more realistic surrogate for low-level contaminant concentrations than iodine. Comparing trace capacity number with chloroform capacity yields a much better correlation than iodine number (see figure 2).To demonstrate the comparison of Iodine Number and Trace Capacity Number with respect to TTHM removal, two activated carbons were selected and tested side-by-side in a 1-inch column study. The purpose of a column study is to obtain the breakthrough curve under dynamic conditions, which show how the concentration of the contaminants in the effluent will vary with the volume of liquid treated.
The rate at which the contaminants are adsorbed by the carbon can only be determined by dynamic column tests. This is the key to the system design.
The first activated carbon selected was a commercially available bituminous coal-based carbon that had not been optimized for trace capacity. The second carbon selected, also commercially available, was optimized for Trace Capacity Number. Table 1 lists the properties of each carbon tested. Both carbons have similar Iodine Numbers, but vary with regard to Trace Capacity Number. The water utilized in the column study was tap water containing influent levels of chloroform that averaged 6 ppb, and TTHM levels that averaged 92.8 ppb.
In this study, the activated carbon optimized for trace removal outperformed the standard bituminous carbon in the dynamic test. The trace removal carbon has only breakthrough for chloroform whereas the bituminous is showing chloroform and bromodichloromethane breakthrough. Choosing a carbon with a higher Trace Capacity Number yields improved "real world" performance for TTHM removal and leads to lower operating costs for the customer as a result of less frequent carbon changeouts.
Other factors to consider
As with all applications, other factors also come into play besides adsorption capacity when applying activated carbon for TTHM removal. Kinetic factors are also important and need to be taken into account when designing a carbon system.
For most point-of-entry applications, a 12x40 mesh size is most commonly used; contact times in the range of five to seven minutes per bed are standard when this particle size is applied. Typically, two beds operating in series are recommended to optimize the utilization of the carbon and ensure that breakthrough is contained.
In cases where space limitations exist and pressure drop is not a factor, a 20x50 mesh size carbon can be applied using a shorter contact time of two to three minutes, allowing for smaller beds to be used.
Other water characteristics that can impact the life of the activated carbon must also be taken into account when applying carbon for TTHM removal. Background organics, often measured as total organic carbon (TOC) can competitively adsorb and shorten the bed life of the carbon, and inorganics, such as iron, can precipitate onto carbon beds and impact carbon performance.
Proper monitoring and backwashing of carbon beds is recommended to ensure that the carbon is working properly and that the system is maintained.
While chlorination of drinking water has its benefits, it also has its drawbacks through the formation of TTHMs. Fortunately, water treatment professionals have tools at their disposal to deal with TTHM problems in their water supply. Activated carbon, when properly selected and applied, is one of those tools.
close
www.h2o-china.com论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 [email protected] ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








