Disinfection with Chlorine Dioxide
| 论文类型 | 技术与工程 | 发表日期 | 2005-10-01 |
| 作者 | Gerald,Cowley | ||
| 摘要 | A number of methods for controlling disinfection by-products (DBP‘s) are being implemented in North America in order to comply with the new U.S. EPA Stage 1 Safe Drinking Water Act (SDWA). These include the use of granulated active carbon (GAC), enhanced | ||
Disinfection with Chlorine Dioxide
Gerald Cowley
Market Development Director
(Sterling Pulp Chemicals Toronto, Ontario M9B 6C7)
Abstract A number of methods for controlling disinfection by-products (DBP‘s) are being implemented in North America in order to comply with the new U.S. EPA Stage 1 Safe Drinking Water Act (SDWA). These include the use of granulated active carbon (GAC), enhanced coagulation (EC), and also the use of alternative disinfectants. Due to differences in water quality across North America, no single approach will fit every location. It is critical therefore, that Water Utilities have as many options as possible for addressing the disinfection versus disinfection by-products dilemma.
One of these options is the use of chlorine dioxide (ClO2) . In the past, principally due to its cost, it was used only for odor and taste problems. However, recent studies have confirmed that it is a very effective disinfectant, and that the concerns about its specific DBP‘s (chlorite and chlorate) can and are being avoided.
Introduction to chlorine dioxide
Chlorine dioxide (ClO2) was first used in North American water treatment during the 1940‘s in Niagara Falls, N.Y. (1). Their water source was heavily contaminated with chlorophenols, formed during pretreatment with chlorine (Cl2). Chlorine dioxide has the ability to oxidize them. Its disinfection properties were also recognized, but the major advantage was in taste and odor.
In Canada in the late 1940‘s, ClO2 was introduced to the Pulp and Paper Industry by Dr. Howard Rapson of the University of Toronto, to produce very high strength and high brightness paper, a combination never seen before. In the 1980‘s, Dr. Rapson was also the first to recognize that ClO2 did not form mutagenic liquid effluent discharges in Pulp Mill effluents . This has resulted in the almost complete replacement of chlorine with ClO2 in North America and elsewhere for bleaching. The effect on decreasing pollution from Pulp Mills can be seen in the following Graphs 1 through 3:
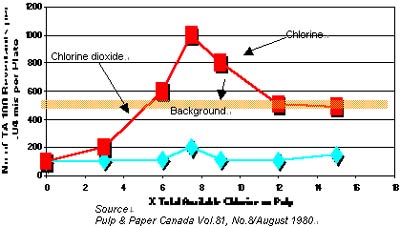
Graph 1. No. of Revertants in Bleach Filtrate vs Available Chlorine
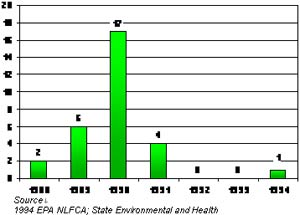
Graph 2 Yearly Number of Waterbodies Placed under a Dioxin Advisory
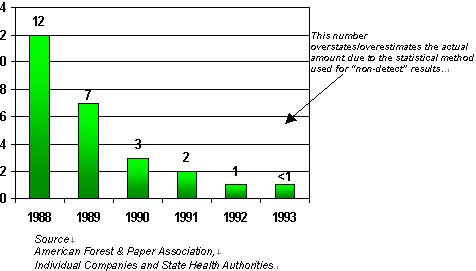
Graph 3 Dioxin Discharges into U.S Pulp Mill Waters. 2,3,7,8 TCDD Equivalents.
The fact that ClO2 oxidizes organics is the fundamental reason why its by-products are less toxic. Also, ClO2 is less reactive than chlorine or ozone (O3), with less of its power being consumed by oxidizing fiber (or other organic and inorganic species). Thus in the last fifty years, ClO2 use has grown from a small specialty chemical with its use measured in pounds per day worldwide to over a million tonnes per year in 1999.
Recent developments with chlorine dioxide in water treatment.
Toxicity
Chlorine dioxide is probably the most extensively tested disinfectant in history. This is because not much was known about the chemical, whereas experience with chlorine extended in practice for over a hundred years. It is likely that in the light of this research, ClO2 will be seen as one of the least hazardous of residual disinfectants. There is growing evidence that chlorite ion, which is the principal by-product of ClO2, is itself an adequate post-disinfectant at levels normally used in drinking water (3). This was previously suggested by Masschelein (4). Even when residual ClO2 had decayed to non-detectable levels, by-product chlorite prevents any regrowth of microbes within the system. There is growing evidence that chlorite also reduces biofilms. In 1994 the Chemical Manufacturers Association (CMA) and the U.S. EPA agreed to CMA‘s conducting a two-generation reproduction neurological development study on rats. This was completed in 1997, and as a result, a No Observable Effect Level (NOEL) was established for chlorite ion of 0.03mg/kg/day (5). From this, the Maximum Contaminant Level (MCL) for ClO2 and chlorite are set at 0.8mg/L and 1.0mg/L respectively, in the Safe Drinking Water Act of November 1998. The Study will be published later this year, but is available through the CMA. A copy has been sent to Health Canada for consideration in the new Canadian Regulations expected in the near future. In 1998, a Swedish Medical Birth Registry and Official data on Drinking Water Disinfection Method study was conducted in Sweden (6). Data for approximately 74,000 births were compared. Three areas were studied, the first using ClO2 , the second, sodium hypochlorite, and the third, no disinfection .No statistically significant differences were determined between births in the area using ClO2 and the area where no disinfection was practiced.
Operations
One of the difficulties experienced by Water Treatment Plants who use ClO2 is the complexity and sensitivity of the method of analysis. The two EPA approved procedures are Amperometric and DPD Titration methods. Both require highly skilled analysts and can take upwards of 40-45 minutes per sample. In fact, in a survey of Water Plants who had tried ClO2 and then abandoned its use, the majority gave this as the principal reason.
Research was carried out in Toronto and in France to find better and faster methods (7,8). An improved hand held unit is now on the market, which will give a ClO2 concentration value within 2-3 minutes of obtaining the sample, and can be used in the field (9). This unit will be field tested in Ontario this autumn.
Disinfection By-products.
ClO2 does not react with Natural Organic Matter (NOM) to produce either Tri-Halomethanes (THM‘s) or Halo-acetic acids (HAA‘s) at doses used in drinking water (10).
ClO2 does not react with bromides to produce Bromate (10), which is a known carcinogen, and has an MCL of 10ppb (11). This can be readily understood by reference to the following table:
Table 1 Oxidation Potentials of Various Water Treatment Agents
Source: Courtesy of R.I.C.E. International. * Agents with excellent disinfection capabilities.
Disinfection using Chlorine Dioxide
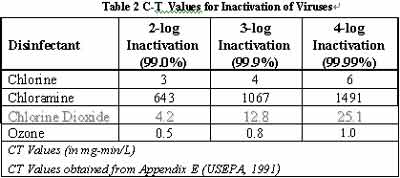
For disinfection against viruses, ClO2 is not as fast as chlorine, but CT values are nevertheless practical.
Giardia, ClO2 is five times more effective than chlorine
Table 3 C-T Values for the Inactivation of Giardia Cysts @ 10Deg.C and pH 6-9
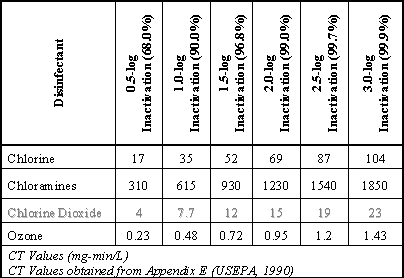
U.S. EPA calculated the relative costs of compliance with 1998 SDWA Stage 1, showing that ClO2 is very cost effective. The data is shown in the following graph:
Graph 4 Cost of Compliance with SDWA Stage 1
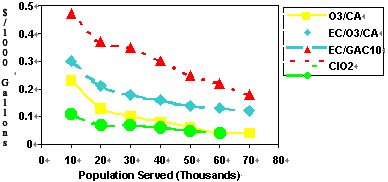
For disinfection against cryptosporidium, ClO2 has been tested extensively by Dr. Gordon Finch at the University of Alberta. For 1-Log Kill, CT values in the range 100-120 are needed at temperatures between 5-15 deg.C.and pH between 6.5-8.5. At very low temperatures, ClO2 is not effective. However, despite this, ClO2 can be the most economical disinfection choice depending upon system size, TOC, temperature and pH.(12).
Table 4. Approximate CT Values for Cryptosporidium Inactivation
Experimental
Conditions
Ozone
Chlorine Dioxide
Log Units of Inactivation
Log Units of Inactivation
pH
Temp.
0.5
1.0
2.0
0.5
1.0
2.0
8.5
15
1.7
3.5
7.0
30
55
125
8.5
5
2.3
4.3
8.5
45
100
275
8.5
15
1.7
3.5
7.0
35
120
225
6.5
5
2.3
4.3
8.5
100
350
1000
Dr. Finch also demonstrated synergistic benefits of ozone/ClO2 and ClO2/chlorine
Chlorine dioxide generator development
ClO2 is produced on site using a generator. Typically three types of generators are in general use, using a choice of three different feeds :-
Using chlorine gas and sodium chlorite:
Using chlorine gas and sodium chlorite:
NaClO2 + 1/2 Cl2 —> ClO2 + NaCl + Excess Chlorine (1)
Using hydrochloric acid and sodium chlorite:
5NaClO2 + 4HCl—>4ClO2 + 2H2O + 5NaCl + Excess acid (2)
Using hydrochloric acid, sodium hypochlorite and sodium chlorite:
2NaClO2 + HOCl + HCl —>2ClO2 + 2NaCl + H2O + Excess Chlorine (3)
A typical generator is shown in the following photograph:

In all of these cases, the product ClO2 is not pure. Excess chlorine is needed to drive the reaction to completion . This excess also reacts to form chlorate (ClO3-) via the following side reactions :
ClO2- + HOCl —> ClO3- +Cl- + H+ (4)
ClO2- + Cl2 + H2O—> ClO3- + 2Cl- + H+ (5)
ClO2 + 1/2Cl2 + H2O—>ClO3- + Cl- + 2H+ (6)
A survey of field generators was conducted in 1998-1999, which shows the dependence of generator efficiency and excess chlorine:
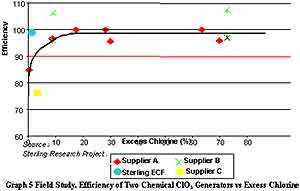
Although chlorate is not considered toxic, the loss in efficiency can be significant (13).
These reactions will occur also in the treated water, so that the efficacy of disinfection is lower. The conclusion is that ClO2 for disinfection should be as pure as practically possible.
In an effort to solve this problem, single chemical generators have been developed using an electrochemical cell as the "Reducing Agent", along with a perstraction membrane to separate pure ClO2. The overall reaction is as follows :-
NaClO2 + H2O —> ClO2 (Anode) + NaOH (Cathode) + 1/2H2 (Cathode) (7)
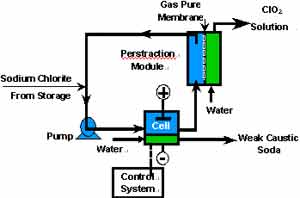
Diagram 2 Perstraction System for Producing Pure Chlorine Dioxide.
The purity of the product ClO2 (over 99%) can be seen in Graph 5. The perstraction membrane will only pass components which exert vapor pressure. This includes water vapor and ClO2, but not chlorite, chlorate or chloride. The product pH is 6.5, very close to that of pure ClO2 (14).

Diagram 3 ECF Module
This system was developed in the laboratory in Toronto, and will be field tested in Ontario in the autumn of 1999.
REFERENCES
1. Gates: The Chlorine Dioxide Handbook AWWA
2. Cowley,Kaczur,Ranger Lipsztajn."Recent Advances in Chlorine Dioxide Application Technology." WWW.ClO2.com.
3. Elf-Atochem. "Chlorine Dioxide By-Products Formation and Reactivity in the Distribution System.
4. Masschelein:"Chlorine Dioxide"pp157 .Ann Arbour Science.
5. Federal Register. 40 CFR Parts 9 141 142, pp 69403, 7. Nov 1998
6. Kullen. "Drinking Water and Delivery Outcome" Second European Symposium on Chlorine Dioxide and Disinfection. Paris June 24/25 1999.
7. Hofman.Univ.Toronto. Env.Technology,vol 19.1998
8. Lapoire, "Evaluation of a Specific Chlorine Dioxide Method intended to Onsite Use" Second European Symposium on Chlorine Dioxide and Disinfection. Paris June 24/25 1999.
9. Hach Company. http://www.hach.com.
10. Korn et Al. Univ. Toronto. AWWA Annual Conference, Chicago June1999.
11. Federal Register. 40 CFR Parts 9 141 142 pp 69405. Nov 1998
12. Gregory Dean. "Applicability of Chlorine Dioxide for Cryptosporidium Inactivation. WQTC. San Diego Nov.1998.
13. Greise et al. AWWA Journ.Nov 1992.
14. Cowley et al. US Patent 5,932,085 Aug 1999
close
www.h2o-china.com论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 [email protected] ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








