Control of THMs with Chlorine Dioxide
| 论文类型 | 技术与工程 | 发表日期 | 2005-10-01 |
| 作者 | John,Newlove,Jennife | ||
| 摘要 | Chlorine dioxide (ClO2) is effective as both a disinfectant and an oxidant in potable water and wastewater treatment. Its selective reactivity makes chlorine dioxide a powerful oxidizing agent useful in many water treating applications for which chlorine | ||
Control of THMs with Chlorine Dioxide
John Newlove Jennifer Miller, Ph.D. Greg D. Simpson, Ph.D.
(Vulcan Performance Chemicals)
ABSTRACT Chlorine dioxide (ClO2) is effective as both a disinfectant and an oxidant in potable water and wastewater treatment. Its selective reactivity makes chlorine dioxide a powerful oxidizing agent useful in many water treating applications for which chlorine and other oxidizing agents are unsuitable. Unlike chlorine, chlorine dioxide does not react with naturally occurring organic materials to form trihalomethanes (THMs). Chlorine dioxide aids in reducing the formation of Total Trihalomethanes (TTHMs) and haloacetic acids (HAA) by oxidizing THM precursors.
The US EPA‘s National Primary Drinking Water Regulations1 set a maximum contaminant level (MCL) of 0.08 mg/L for TTHM. The EPA has identified chlorine dioxide as an alternative or supplemental oxidant-disinfectant that is one of the most suitable for TTHM treatment and control2.
This paper provides a brief overview of THM formation and the use of chlorine dioxide for preventing or controlling THMs. Two case histories are presented.
1. INTRODUCTION
An estimated 12 million people in the United States are served with drinking water disinfected by chlorine dioxide generated from sodium chlorite. Chlorine dioxide is highly effective in controlling waterborne pathogens while minimizing halogenated disinfection Byproducts in drinking water. Chlorine dioxide is a broad-spectrum microbiocide as effective as chlorine against viruses, bacteria, and fungi, and more effective than chlorine for the inactivation of the encysted parasites Giardia and Cryptosporidium. Chlorine dioxide is also an effective control strategy for taste, odor, color, iron, and manganese removal.
1.1 Disinfection
Under US National Primary Drinking Water Reguations3, the disinfection treatment must be sufficient to ensure at least a 99.9 percent (3-log) removal and/or inactivation of Giardia lamblia cysts and 99.99 percent (4-log) removal and/or inactivation of enteric viruses. At the CT values necessary for chlorine dioxide to inactivate 99.9 percent of Giardia lamblia cysts, the simultaneous inactivation of 99.99 percent of enteric viruses is also assured. In addition, the USEPA recently published the Stage 2 Microbial and Disinfection Byproducts Federal Advisory Committee Agreement in Principle4 which states that chlorine dioxide is an acceptable treatment approach for management of Cryptosporidium.
Chlorine dioxide is also as effective as chlorine in destroying coliform populations in wastewater effluents, and is superior to chlorine in the treatment of viruses commonly found in secondary wastewater effluents5.
1.2 Trihalomethanes (THM)
The reactions of chlorine dioxide with humic substances (THM precursors) do not result in the formation of THMs. Pretreatment with chlorine dioxide oxidizes THM precursors, which are removed during coagulation, settling, and filtration before final disinfection. When chlorine is used as the final disinfectant this modification of standard chlorination practices can result in a 50-70% decrease in THMs in the finished water.
1.3 Taste and Odor
Chlorine dioxide has been shown to be effective at a dosage of 1mg/L in the control of taste and odors resulting from algae and decaying vegetation. Chlorine dioxide effectively oxidizes low- threshold odor compounds, including geosmin, 2,3,6-trichloroanisole (TCA), 2 methyl-isoborneal (MIB), and members of the pyrazine family6. Chlorine dioxide has also been proven effective in destroying taste and odor-producing phenolic compounds, which are oxidized with minimal production of chlorophenol. Oxidation of other off-taste and odor-causing compounds, such as mercaptans and disubstituted organic sulfides has been demonstrated7.
1.4 Iron and Manganese
Iron (Fe) and manganese (Mn) exceed recommended secondary maximum concentration levels in roughly 40% of the public water supplies in the United States. These recommended levels of 0.3 mg/L Fe and 0.05 mg/L Mn correspond to approximate concentrations at which iron and manganese will cause aesthetic problems such as colored water, turbidity, staining, and taste. Iron and manganese may also accelerate biological growths in the distribution system, further exacerbating taste, odor, and color problems. Chlorine dioxide reacts rapidly with soluble forms of iron and manganese to form precipitates that can be removed through sedimentation and filtration. Preoxidation with chlorine dioxide also improves coagulation and settling, resulting in better filter-run times8.
2. THM FORMATION
In the mid to late 70‘s, researchers suggested a possible link between chlorination of potable water and increased cancer mortality rates9,10. The potential increase in cancer rates was tied to the production of trihalomethanes, THMs11,12. The USEPA established 0.1 mg/L as the maximum THM containment level for drinking water. Research in the area of THM reduction in potable water1,13-15 led the EPA in 1983 to cite chlorine dioxide as an effective means of controlling THMs.
Further research indicated that chlorination of surface water, particularly water which was contaminated with high levels of humic and fulvic acids, resulted in significant levels of halogenated methanes. Since analytical techniques were not available at the time to speciate the various forms, these were considered together as Total Trihalomethanes, TTHMs. As analytical techniques have improved, further speciation of these individual THMs became possible. The identification of THMs in chlorinated water supplies led to concerns over their potential health effects including reproductive effects and the classification of chloroform, bromodichloromethane and certain other disinfection byproducts (DBPs) as carcinogens.
Table 1. MCL and MCLG1 for Disinfection Byproducts
Disinfection Byproducts
MCLG (mg/L)
MCL (mg/L)
Total Trihalomethanes (TTHMs)
N/A
0.080
- Chloroform
0
-
- Bromodichlormethane
0
-
- Dibromochloromethane
0.6
-
- Bromoform
0
-
Haloacetic Acids (five)
N/A
0.060
- Dichloroacetic acid
0
-
- Trichloroacetic acid
0.3
-
Bromate
0
0.010
Note 1: MCLG is the maximum contaminant level goal.
The final Disinfectants and Disinfection Byproducts Rule1 (DBPR) set a maximum contaminant level (MCL) of 0.08 mg/L for total trihalomethanes (TTHM) in drinking water, and extended the MCL to all size systems. In addition, a new MCL of 0.06 mg/L was established for haloacetic acids (HAA5). Current limits are shown in Table 1.
3. CHLORINE DIOXIDE TREATMENT OF THMS
In contrast with chlorine, it is now believed the reactions of chlorine dioxide with humic substances (the precursors of trihalomethanes) do not result in the formation of THMs. (Although low levels have sometimes been produced, it is now thought that these result from trace amounts of chlorine impurities in the generated ClO2.)
In 1976, Miltner demonstrated that chlorine dioxide produces THMs only at extremely low levels when used as a drinking water disinfectant16. This was later borne out in field studies at Evansville, Indiana17; Hamilton, Ohio18; Galveston, Texas19; Louisville, Kentucky20; Davenport Iowa21; and Contra Costa, California14. These case studies demonstrate the versatility and effectiveness of chlorine dioxide for THM control in municipal water treatment systems.
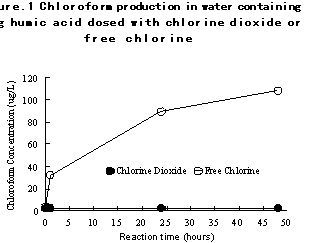
In other work, Noack and Doerr in 1978 reported on the reaction of chlorine and chlorine dioxide and combinations of these oxidants with humic acid at drinking water concentrations22. They reported that chlorine dioxide reacted much slower at drinking water concentrations than they had expected based on previous work at higher concentrations of chlorine dioxide. As expected, reactions with chlorine produced chloroform, while reactions with chlorine dioxide did not produce chloroform. Figure 1 shows their results.
In 1984, Rav-Acha recognized that much of the previous laboratory work on the reaction of chlorine dioxide to produce chlorinated organics, including trihalomethanes, had been done with significantly higher than normal use concentrations15. He reported that no THMs were detected when chlorine dioxide was applied to drinking water under typical treatment conditions, confirming the results of Miltner16, Henderson, et al.23, and Miller, et al13. He concluded that chlorine dioxide produces mainly oxidation byproducts rather than chlorination byproducts. He also noted the greater selectivity of chlorine dioxide by the much lower demand for chlorine dioxide in natural water than for chlorine, and by the relatively constant demand for chlorine dioxide relative to widely varying demands for chlorine in the same water.
Lykins, in 1986, reported on the reduction of trihalomethane by pretreating with chlorine dioxide1. Before treatment with chlorine dioxide, total THM levels were 141 ug/L. After treatment with chlorine dioxide, the THM level was reduced to 1.4 ug/L in pilot plant data. A GC-MS analysis of the water could not identify any chlorinated by-product that resulted from the feed of chlorine dioxide. These results were duplicated by work at another plant25.
Andrews26, in a recent trial in Canada applied chlorine dioxide for post disinfection. Results showed that TTHMs where reduced from above 30 ug/L when the system was treated with chlorine for post disinfection to below 5 ug/L when the system was treated with chlorine dioxide. HAA‘s were reduced from above 20 ug/L to less than 8 ug/L after post disinfection with chlorine dioxide. In addition, Giardia inactivations also increased from less than 1 log reduction to as much as 3 log reductions. These results also showed that chlorine dioxide was effective at carrying a residual in this distribution system.
In summary chlorine dioxide undergoes only oxidation reactions27,28. Chlorine will oxidize but will also undergo a high percentage of addition/substitution reactions23, resulting in the formation of halogenated organics such as THMs. While many treatment methods have been developed to remove THMs once they have been formed by chlorine treatment, chlorine dioxide treatment is superior in preventing or substantially reducing their initial formation.
3.1 Feed Requirements
Chlorine dioxide is typically applied at a concentration between 0.1 and 5.0 mg/L in the raw water. For most municipal and other potable water systems a chlorine dioxide residual concentration up to 2.0 mg/L is sufficient to provide adequate disinfection. Chlorine dioxide is also applied in the finished water to maintain a distribution system residual. The required dosages will vary with source water conditions, the severity of contamination, and the degree of control required. Residual disinfectant and disinfectant byproducts must be monitored in the US as required by the National Primary Drinking Water Regulations (40 CFR Part 141) and state drinking water standards. For wastewater and sewage applications, residual chlorine dioxide concentrations up to 5 mg/L are generally adequate to achieve the required disinfection.
3.2 Method of Feed
Chlorine dioxide is a gas produced by activating sodium chlorite with an oxidizing agent or an acid source. Sodium chlorite is converted to chlorine dioxide through a chlorine dioxide generator and applied as a dilute solution. Chlorine dioxide solutions should be applied to the processing system at a point, and in a manner which permits adequate mixing and uniform distribution. The feed point should be well below the water level to prevent volatilization of the chlorine dioxide. Sodium chlorite should not be applied directly to potable water.
3.3 Chlorine Dioxide Analysis
In addition to other monitoring requirements, the DBPR also requires that drinking water systems using chlorine dioxide for disinfection or oxidation must monitor their system for chlorine dioxide and chlorite. Although these exact limits and methods may not be appropriate in other countries, they are included here for completeness.
Chlorine Dioxide: For US compliance monitoring for residuals of chlorine dioxide, one of the two approved methods specified in 40 CFR §141.131(c) must be used:
DPD Method, 4500-ClO2 D, and Amperometric Method II, 4500-ClO2 E29.
Where approved by the state, systems may also measure residual disinfectant concentrations of chlorine dioxide by using DPD colorimetric test kits.
Sodium Chlorite: For compliance monitoring for chlorite, water systems must use one of three approved methods specified in 40 CFR §141.131(b):
Amperometric Method II, 4500-ClO2 E
Ion Chromatography, EPA Method 300.030, or
Ion Chromatography, EPA Method 300.131.
These regulations further specify that Amperometric Titration may be used for routine daily monitoring of chlorite at the entrance to the distribution system, but that Ion Chromatography must be used for routine monthly monitoring of chlorite and for additional monitoring of chlorite in the distribution system.
4. CASE HISTORIES
4.1 Case History One: Use of Chlorine Dioxide Improves Taste and Odor and Controls THMs
In one central US municipality, between 12 and 36 million gallons per day of drinking water are disinfected. The plant experienced well over 100 taste and odor complaints per day. Chlorine gas was used as the primary disinfectant for the raw surface water entering the plant. This plant produced relatively high levels of THMs in the disinfected drinking water. An alternative primary disinfectant was required to reduce the number of taste and odor complaints, inhibit THM formation, and adequately disinfect the drinking water.
A recommendation was made to the municipality to treat the raw water with chlorine dioxide, using a two-chemical flow-paced Rio Lindaò generator, at a feed rate of 1.75-2.0 mg/L. The injection point was moved 2,500 ft up the raw water line to minimize taste and odor and achieve disinfection credit.
The chlorine dioxide program significantly reduced the number of taste and odor complaints to less than 2/month and effectively lowered THM levels in the disinfected drinking water to well below the USEPA limits. Use of chlorine dioxide also allowed the plant to claim disinfection credit. The program has been in place since 1998 and the customer continues to be extremely satisfied with the results. A plot of both THM and taste and odor complaints both before and after implementation of the ClO2 program is shown in Figure 2.
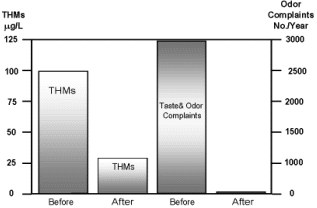
Figure 2 THM and Taste & Odor Complaints Before and After ClO2 Program Implementation
4.2 Case History Two: Use of Chlorine Dioxide to Control THMs in Municipal Drinking Water
In this southeastern US municipality, 33 million gallons per day of drinking water were disinfected. Chlorine gas was used as the primary disinfectant for the raw water entering the plant. This plant produced relatively high levels of THMs in the disinfected drinking water. An alternative primary disinfectant was required to prevent the formation of elevated levels of THMs while adequately disinfecting the drinking water.
A recommendation was made to the municipality to treat the raw water with chlorine dioxide, using a two-chemical Rio Lindaò generator, at a feed rate of 0.5 mg/L. The continued use of chlorine gas as a final disinfectant prior to distribution was also recommended.
Chlorine dioxide effectively lowered THM levels in the disinfected drinking water to well below the levels required by the USEPA. Additional benefits resulted from the use of chlorine dioxide: control of iron and manganese, and oxidation of substances known to produce adverse taste and odor in the disinfected drinking water. The program has been in place since 1992 and the customer continues to be extremely satisfied with the results. A plot of the THM levels before and after implementation of the chlorine dioxide program is shown in Figure 3.
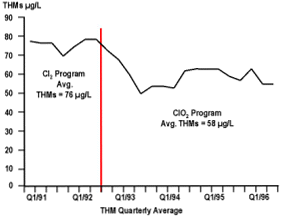
Figure 3 THM Levels Before and After Implementation of ClO2 Program
4.3 CONCLUSIONS
Chlorine dioxide has been demonstrated to be an effective control strategy to prevent and or reduce the formation of THMs in potable water treatment. In addition, chlorine dioxide has been demonstrated as an effective control strategy for pre- and post disinfection of potable water, taste and odor control and iron and manganese removal.
Chlorine dioxide is not only economical, but is also effective in both preoxidation and disinfection as a replacement for prechlorination and post disinfection. For this reason, the EPA has identified chlorine dioxide as an alternative or supplemental oxidant-disinfectant that is one of the most suitable for TTHM treatment and control2.
REFERENCES
1. Federal Register, National Primary Drinking Water Regulations: Disinfectants and Disinfection Byproducts, 63 FR 69389 (December 16, 1998).
2. USEPA, "Alternative Disinfectants and Oxidants Guidance Manual " EPA/815/R-99/014, (April 1999).
3. Code of Federal Regulations, National Primary Drinking Water Regulations, Subpart H - Filtration and Disinfection. 40 CFR 141.70-.75.
4. Federal Register, Stage 2 Microbial and Disinfection Byproducts Federal Advisory Committee Agreement in Principal (Stage 2 M-DBP Agreement), 65FR83015, (December 29, 2000).
5. Roberts, P.V., Aieta, E.M., Berg, J.D., and Chow. B.M., "Chlorine Dioxide for Wastewater Disinfection: A Feasibility Evaluation," Stanford University, EPA-600/2-81-092 (1981).
6. Lalezary, S., Pirbdzdri, M., Mc.Guire, M.J., "Oxidation of Five Earthy-Musty Taste and Odor Compounds", JAWWA, 62-69, (March 1986).
7. Warf, C.C. Jr., "Chlorine Dioxide and The Small Drinking Water System", published in Providing Safe Drinking Water in Small Drinking Water Systems, Lewis Publishers, 121-131 (1998).
8. Stevens, A.A., "Reaction of Chlorine Dioxide", Environ. Health Perspect., 46;101 (1982)
9. Alavanja, M., Goldstein I., and Sasser, M., "A Case Control Study of Gastrointestinal and Urinary Tract Cancer Mortality and Drinking Water Chlorination, in Water Chlorination," in Environmental Impact and Health Effects, (edited by Jolley, R. L.), Vol. 2, pp. 395. Ann Arbor Science, Ann Arbor, MI., 1980.
10. Page, T., Harris, R. H., and Epstein, S. S., "Drinking Water and Cancer Mortality in Louisiana," Science 193, 55 (1976).
11. Report of the Carcinogenesis Bioassay of Chloroform. NTIS PB264018/AS, National Cancer Institute, 1976.
12. Roe, F. J. C., "Preliminary Report of Long-Term Tests of Chloroform in Rats, Mice and Dogs," unpublished Report. Cited in Ozone, Chlorine Dioxide and Chloramines as alternatives to Chlorine for Disinfection of Drinking Water. Water Supply Research, Office of Research and Development, U.S. EPA, Cincinnati, OH, 1976.
13. Miller, G. W., Rice, R. G., Robson, C. M., Kuhn, W., and Wolf, H., "An Assessment of Ozone and Chlorine Dioxide Technologies for Treatment of Municipal Water Supplies," EPA Report 600012-78-147, Cincinnati, OH, 1978.
14. Lange, A., and E. Kawczynski, 1978, "Controlling Organics, the Contra Costa County Water District," JAWWA 70:653.
15. Rav-Acha, Ch., 1984, "The Reactions of Chlorine Dioxide with Aquatic Organic Materials and Their Health Effects," Water Res., 18(11):84.
16. Miltner, R. J., 1976, "The Effect of Chlorine Dioxide on Trihalomethanes in Drinking Water," MS Thesis, University of Cincinnati.
17. Lykins, B.W., Jr. and Griese, M.H., "Using Chlorine Dioxide for Trihalomethane Control," JAWWA, 78, 88 (1986).
18. Augenstein, H.W., "Use of Chlorine Dioxide to Disinfect Water Supplies," JAWWA, 66, 716 (1974).
19. Myers, G.L., et al., "Control of Trihalomethanes and Taste and Odor at Galveston County Water Authority," Proc. Annual Meeting, AWWA, at Denver, CO, 1667-1675 (1986).
20. Hubbs, S. A., M. Guers, and J. Siria, 1980, "Plant-scale Examination and Control of a ClO2-Chloramination Process at the Louisville Water Company," in Water Chlorination: Environmental Impact and Health Effects, Vol 3., Jolley, R. L., et. al., eds, Ann Arbor Science Publishers, Ann Arbor, Michigan.
21. Blanck, C., 1979, "Trihalomethane Reduction in Operating Water Treatment Plants," JAWWA 71:525.
22. Noack, M. G., and R. L. Doerr, 1978, "Reactions of Chlorine, Chlorine Dioxide and Mixtures Thereof with Humic Acid: An Interim Report.," in Water Chlorination: Environmental Impact and Health Effects, Vol. 2., (R. L. Jolley, et. al., editors), Ann Arbor Science. Ann Arbor, MI.
23. Henderson, J. E., G. R. Peyton, and W. H. Glaze, 1976, "A Convenient Liquid-Liquid Extraction Method for the Determination of Halomethanes in Water at the ppb Level," in Identification and Analysis of Organic Pollutants in Water, edited by Keith, L. H., Ann Arbor Science, Ann Arbor, MI., 105.
24. Lykins, B. W., and M. H. Griese, June 1986, "Using Chlorine Dioxide for Trihalomethane Control," JAWWA 78:6:88.
25. Lykins, B. W., and W. Koffskey, August 1985, "Products Identified at an Alternative Disinfection Pilot Plant," 2nd International Symposium on Health Effects of Drinking Water Disinfectants and Disinfection Byproducts, Cincinnati, OH.
26. Andrews, R. et al., "Chlorine Dioxide Trial as a Post Disinfectant in Wiarton, Ontario, Fourth Int. Symp. International Symposium on Chlorine Dioxide, February 15-16, Las Vegas, Nevada (2001).
27. Synan, J. F., 1979, "Chlorine Dioxide - An Effective Biocide for Recycled or Reused Water Systems," Trans. Citrus Eng. Conf., 25:66.
28. Rav-Acha, Ch., and R. Blits, 1985, "The Different Reaction Mechanisms by Which Chlorine and Chlorine Dioxide React with Polycyclic Aromatic Hydrocarbons (PAH) in Water," Water Research, 19(10):1273.
29. Standard Methods for the Examination of Water and Wastewater, APHA, AWWA and WEF, Washington, D.C. (20th Ed. l998).
30. Methods for the Determination of Inorganic Substances in Environmental Samples. USEPA. 1993. EPA/600/R-93/100.
31. USEPA Method 300.1, Determination of Inorganic Anions in Drinking Water by Ion Chromatography, Revision 1.0. USEPA. 1997. EPA/600/R-98/118
close
www.h2o-china.com论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 [email protected] ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








