Evaluation of Microbial Community Structure and Purification
| 论文类型 | 技术与工程 | 发表日期 | 2003-11-01 |
| 来源 | 第三届环境模拟与污染控制学术研讨会 | ||
| 作者 | Udin,Hasanudin,Tadao | ||
| 摘要 | UdinHasanudin(1),Tadao Kunihiro(2), Masafumi Fujita(3),Hong-Ying Hu(4),Koichi Fujie(1)and Teruaki Suzuki(5) [1] Department of Ecological Eng., Toyohashi Universityof Technology, Tempaku-cho 441-8580, Japan. 2 Fac | ||
UdinHasanudin(1),Tadao Kunihiro(2), Masafumi Fujita(3),Hong-Ying Hu(4),Koichi Fujie(1)and Teruaki Suzuki(5)
[1] Department of Ecological Eng., Toyohashi Universityof Technology, Tempaku-cho 441-8580, Japan.
2 Faculty of Environmental and Symbiotic Sciences, PerfecturalUniversity of Kumamoto 3-1-100 Tsukide, Kumamoto City 862-8502, Japan.
3 Department of Civil and Environmental Engineering, University of Yamanashi, 4-3-11 Takeda, Kofu, Yamanashi 400-8511, Japan.
4 Department of Environmental Science and Engineering, Tsinghua University, Beijing, 100084
5 Aichi Fisheries Research Institute, 97, Wakamiya, Miya-cho, Gamagori, 443-0021, Japan.
Abstract
Evaluation ofchanges in microbial community structure and purification capacity in tidal flat sediment with and without clams are important in order to clarify the relationship between clams and microorganisms on the improvement of tidal flat purification function. The objectives of this research are to evaluate the effect of clam on changes in microbial community structure and purification capacity of tidal flat sediments.Mesocosm of artificial tidal flat sediment with and without clams in AichiFisheries Research Institute, Gamagori, Aichi, Japan were employed in thisexperiment. It was found that clams increased diversity, number of quinonespecies, and density of menaquinone (MK) containing bacteria intidal flat sediment. Diversity of quinone profile increased from about 9 atinitial condition to about 11.6 after4-month clam acclimations. Number of quinone species also increased from 12 to15 at the same time. Increases in biodegradability of organic matter and changes in chemicalsenvironment caused changes in microbial community structure in tidal flat sediment with clams. NH4-N concentration in tidalflat sediment with clams was about 3 times higher than in the sediment without clams. This condition was suitable for nitrifyingbacteria growth and increase nitrification process in tidal flat sediment withclams. High activity of nitrifying bacteria produced higher concentration of NO3-N.Also, clam activities and high concentration of biodegradable compoundsdepleted dissolved oxygen (DO) concentration in the sediment. This conditionwas suitable for MK-containing bacteria growth. High concentration ofMK-containing bacteria and low concentration of DO were suitable condition forNO3-N removal from tidal flat sediment. NO3-N removal capacityin upper layer of tidal flat sediment with clams was about 3 times higher than inthe sediment without clams.
Keywords: biodegradability; clams;microbial community structure; nitrate removal; quinone; tidal flat.
1. Introduction
Tidal flat is critical transition zonebetween land, freshwater habitats, and the sea [1].This place wasrecognized as important sites for nutrient transformation and sequestration viabiogeochemical cycling [2, 3].Population growth hasdrastically increased during the last century, and since coastal areas areintensively inhabited, pollution pressure on marine environments has beendramatically enhanced over this time [4].Overload of nutrient to tidal flat area will cause nutrient accumulation and finallycausing malfunction of tidal flat on seawater purification. These conditionspromote eutrophication and often cause phytoplankton blooms followed bydeposition of organic matter and detritus to seabed sediment [5].Eutrophication can be regarded as a contributing orenhancing factor, which together with adverse meteorological and hydrographicalconditions causes depletion of oxygen and subsequent mortality of benthiccommunities [4]. Thelarge input of organic matter resulted in anoxia within the sediment, as a consequenceof respiration processes, and an enhancement the activities of sulfate-reducingbacteria to produce hydrogen sulfide [5, 6, 7].Oxygen-deficient water further well up onto surface layer, and moved towardsshore, causing a catastrophic impact to the benthic ecosystem of the intertidalflats in the innermost parts of the bay [5].Therefore, the improvement of natural self-purification potential of tidalflat ecosystem isnecessary to enhance nutrient removal from tidal flat area and preventeutrophication.
It has been known that microbes play animportant role in primary production, nutrient cycling and decomposition oforganic matter [8, 9].Also, benthic nutrient regeneration is known as amajor driving force in the dynamics of biophilic elements in coastal marineecosystems. Macrozoobenthos play a fundamental role in nutrient regeneration throughtheir excretory products. One of theimportant macrobiotic in tidal flat is clam (Tapes philippinarum). Tapesphilippinarum is the major contributor species to the macrozoobenthosbiomass in tidal flat [10]. Clams as a suspendedfeeder were recognized to have filtering capabilities as an important role inremoving particulate organic matter (POM) from the water column andconsequently reducing the potential for the occurrence of oxygen-depleted waterdeveloping in the bottom layer [11]. Clams were efficientat removing particle having diameter between 3 – 10 兪m [12]. Also, clam excretion activities producedammonia nitrogen (NH4-N), nitrate (NO3-N), nitrite (NO2-N),total Kjeldahl nitrogen (TKN), phospate (PO4-P), and BOD [10, 13].Thus, the filtering function of clams might produce carbon and nutrient withhigher biodegradability, which useful to enhance microbial growth andaccelerate mineralization process in tidal flat sediment [14].However, uncertainty about the actual contribution of clams to the self-purification capacityof tidal flat sedimentmay still remain due to insufficient data about the symbioticrelationship betweenmicroorganisms and clamsin coastal area. Evaluation of changes in microbial community structure andpurification capacity in tidal flat sediments with and withoutclams are important in order to clarify the relationship between clams andmicroorganisms on the improvement of self-purification function of tidal flatecosystem.
The objectives of this research are to evaluate the effect of clam on changes in microbialcommunity structure and purification capacity of tidal flat sediments. Moreover,the effect of clams on decomposition and biodegradability improvement oforganic matter in tidal flat sediment were also studied to clarify the symbioticrelationship between clams and microorganism on the improvement oftidal flat purification function.
2.Materials and Methods
2.1 Research sites
All experimental plots were located in Aichi Fisheries Research Institute, close to Mikawa Bay, Gamagori, Aichi, Japan. The experiment was conducted in four plots of mesocosms of tidal and subtidal sediments. The subtidal sediment was constructed 15 cm lower than tidal sediment to maintain the sediment always under immersion condition. The dimensions of each plot were 2.0 m (W) x 2.5 m (L) x 0.4 m (D) with sand median grain size of 0.95 mm. Clams with 1.9 cm average length and 1.2 g average whole wet weight (included shell) were added to two plots of these sediments corresponding to about 5000 clams per plot on July 23, 2002. Fresh seawater was pumped from about 200 m offshore and supplied to both sediments in the mesocosms. To synchronized water level with natural condition, the water level in Mikawa Bay was online measured and the data was used to arrange flow rate of seawater to the mesocosms.
2.2Sampling methods
One month after adding the clams, pore water quality in all plots, i.e., DOC and NH4-N were monitored for 24 hours to investigate the effect of clams. Pore water sediment also used to analyze molecular weight distribution. About 20 ml of pore water for each analysis was taken from 5 cm depth of sediment using a syringe with capillary tube equipped with double layers of 1.2 mm filter paper (GF/C filter, Whatman).
Sediment cores (n=2) were collected from the 0 – 10 cm depth of each plot using an acrylic tube (5 cm i.d. x 50 cm length) every month over a year from July 2002. The samples from all plots were immediately cooled by ice and thenstored at –20oC before quinone analysis. The sediment core sample from 0 – 10 cm was also collected from each plot using the same method and immediately used for biodegradability experiment. In order to evaluated NO3-N removal capacities, the sediment core sample collected from 0 – 2 and 9 – 11 cm depth of tidal sediment with (A1; A2) and without (B1; B2) clams. A replaceable ring of acrylic tube with 4 cm I.D. and 2 cm length was used to collect this sample. Sediment samples for NO3-N removal capacity experiment were used immediately after sampling.
2.3Laboratory experiment: experimental set up and procedure
Biodegradability of organic carbon in the sediment was analyzed using modified OECD screening test No. 301E [15]. 5 gram of sediment was extracted with 60 ml of seawater using warring blender (3000 rpm, 10 min) then filtered using 0.3 mm filter paper (GF-75, Advantec). 40 ml of filtered seawater was filled-in to 200 ml of Erlenmeyer flask. Seed and mineral solutions were also added. The flasks were shake and incubated in the dark condition at 22 亇 2oC. Decomposition was followed by DOC analysis at frequent interval over a 28-day period. A control with inoculation, but without either test material or standard, was run in parallel for the determination of DOC blank.
NO3-N removal capacity was investigated in batch laboratory scale. Tidal sediment with 4 cm diameter and 2 cm depth was put in a 100 ml beaker glass. Seawater was filtered with 0.3 mm filter (GF-75, Advantec) to avoid impurities. Sodium nitrate (NaNO3) was used to enrich nitrate concentration in the filtered seawater. The initial NO3-N concentration was set at about 4 mgN·l–1. The enriched seawater for NO3-N removal experiment was purged with nitrogen gas for more than 15 min to make anaerobic condition. 60 ml of the treated seawater was added gently to each beaker glass and was incubated at 30oC. The beaker glass for NO3-N removal experiment was covered with parafilm to prevent oxygen transfer from air to liquid phase. Samples were taken every 3 hours for 12 hours. The flux removal rate (F) was calculated as follow:
F = (Ct–C0)·V/(W·t).............. (1)
where Ct–C0 = the differences between NO3-N concentration at t time and at initial; V = volume of seawater; W = weight of sediment; and t = time.
2.4Analytical methods
The DOC and NH4-N concentrations of pore water were measured using TOC analyzer (TOC-5000A, Shimadzu) and automatic water analyzer (AACS-III, Bran+Luebbee), respectively. T-test statistical analysis was performed to distinguish the differences between pore water quality in the sediment with and without clams. NO3-N was measured using an ion chromatography (DX-120, DIONEX) equipped with Ion Pac column AS14 4-mm (10–32) (DIONEX) and a suppressor of ASRS-ULTRA 4-mm (DIONEX).
Microbial quinones in the sediments were analyzed based on the procedure previously reported [16, 17]. Quinones were first extracted from the sediments by a mixture of chloroform-methanol (2:1, v/v) and then re-extracted by hexane. The crude extract was purified using solid extraction cartridge (Sep-PakÒ Plus Silica, Waters). The types and concentrations of quinones were determined with HPLC equipped with an ODS column (Zorbax-ODS, 4.6 (I.D.) x 250 mm, Shimadzu-Dupont) and a multi-channel UV detector (photodiode array detector, model: SPD-M10A, Shimadzu). A mixture of methanol and di-isopropyl ether (9:2, v/v) was used as the mobile phase at a flow rate of 1.0 ml·min–1. The temperature of the column oven was maintained at 35 oC. Quinone species were identified according to the retention time on the column and the UV spectrum of each peak observed in the multi-channel UV detector. The linier relationship between the logarithm of the retention times of quinones and the equivalent number of isoprenoid unit (ENIU) was also used to identify the quinone type [16, 18]. Ubiquinone with 10 isoprenoid and vitamin K1 were used as the quantitative standards for ubiquinone and menaquinone, respectively. The ENIU can be approximated by the following equation:
ENIUk= a + b log (ETk/ETstd) + c[log(ETk/ETstd)]2 ....... (2)
where ETk representsthe elution time of a quinone species k, and ETstdrepresents the elution time of standard quinone. The constants are shown as a, b, and c, whichare empirically obtained for each HPLC system [19]. The amounts of quinone were calculatedfrom the peak area based on the mole absorption coefficients (ubiquinones: 14.4mM–1cm–1, menaquinones: 17.4 mM–1cm–1and plastoquinones: 15.3 mM–1cm–1) [20]. The quinone mole fraction wascalculated as a ratio of the quinone content in the species k to thetotal quinone content. In thispaper, the abbreviation of quinone types are ubiquinone: UQ, menaquinone: MK, plastoquinone:PQ and vitamin K1: VK1.
Inorder to evaluate the changes of microbial community structure in the sedimentswith and without clams, the diversity (DQ) and dissimilarity (D)indices of respiratory quinone profile were calculated [18]. These indices were calculated by thefollowing equations:
..........(3)
............(4)
where,fk is the mole fraction of quinone species k, nis the number of quinone species with the mole fractions higher than or equalto 0.001, fki and fkj are the molefractions of quinone species k for i and j samples,respectively.
3.Results and Discussion
3.1Organic matter degradation
The average concentrationsof DOC, DTN and NH4-N in pore water of tidal and subtidal sediments withand without clams are shown in Table 1. In tidal sediment with clams, concentrations of DOC and NH4-N were about 1.34 mg/l and 1.14 mg/l higherthan in the sediment without clams, respectively. Clams consumed POM andexcreted their feces as a source of DOC and NH4-N. Most bivalves wereefficient at removing particle having diameter between 3 – 10 兪m and totalsuspended solid removal rate of clams were 0.4–4.9 (mg·l–1 TSS(g-clams) –1 day–1), depend on shell size [12]. The difference between DOC concentrationin the sediment with and without clams was not so high. This strongly suggestsdue to the low concentration of particulate organic carbon (POC) in theseawater. POC concentration in the seawater was only about 2.3–4.9 mg·l–1.Also, sediment microorganisms consumed the DOC produced from clam excretionrapidly. DOC was continuously released from POC and followed by a rapid cyclingof DOC during particle decomposition [21]. NH4-Nconcentration in the sediment with clams was about 80% of DTN. This result is consistent with theresults of previous research.
Table 1. Average concentrations of DOC, DTN, and NH4-N in pore water of tidal and subtidal sediment withand without clams Sediment DOC (mg/l) DTN (mg/l) NH4-N (mg/l) with clams without clams with clams without clams with clams without clams
Tidal Sediment 6.97 5.63 2.15 1.01 1.82 0.54
Subtidal sediment 6.47 5.67 0.51 0.53 0.14 0.04
The excretion rate of T. philippinarum is about 50 mg.(kg.clam)–1day–1and 80 mg.(kg.clam)–1.day–1for ammonia nitrogen and total Kjeldahl nitrogen (TKN), respectively [13]. DOC,DTN and NH4-N concentration in the sediment with clams also higherat low water level. During emersion, thepenetration of oxygen into sediments may increase [22, 23]and lead the production of DOC, DTN and NH4-N due to clam excretion.This result indicated that clams could be used to accelerate degradation oforganic matter and nutrients in tidal flat sediment.
A different pattern was found in subtidal sediment. DOC and NH4-Nconcentrations in pore water of sediment with clams were about 0.8 and 0.1 higher than in the sediment without clams. This indicated that the effect of clams was relatively low. Since subtidal sediment is always under immersion conditions, it is expected that DO concentration should be low, lowering the activity level of clams. Therefore, there was no significant difference in DOC and NH4-N concentrations in subtidal sediment with and without clams. NH4-N concentration in pore water of subtidal sediment with clams was only about 27% of DTN. This also indicated that clam activities were low.
Clams also affected biodegradability of organic matter in tidal flat sediment (Fig. 1). Almost90% of DOC from tidal sedimentwith clams was removed during biodegradability test (within 28 days), while inthe sediment without clams DOC removal was only about 44.2%. This indicated thatclams promoted the production of readily biodegradable substances in tidal flatsediment. Clams consumeparticulate organic matter, digest that compounds in their body, and excretetheir feces as sources of dissolved and readily biodegradable substances. Inthe case of without clams, the degradation of particulate organic matter islikely due to an ecto-enzymatic activity of attached bacteria which renders theparticles soluble through macromolecular hydrolysis and produces dissolvedorganic compounds and small molecules which are subsequently taken up byattached and free-living bacteria [21, 24, 25]. Degradation rate through microbialhydrolysis process is lower than degradation rate through clam activities.Therefore, DOC concentration and biodegradability of organic matter in thesediment with clams were higher than in the sediment without clams.
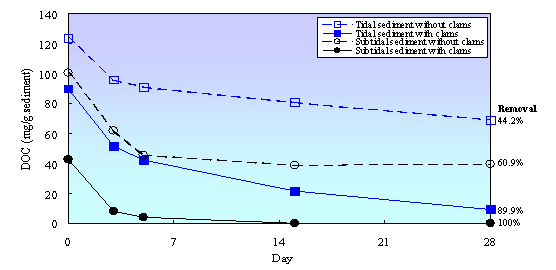
Figure 1. DOC concentration during biodegradability test in tidal flat sediment with and without clams
In subtidal sediment,biodegradability of organic matter in the sediment with clams also much higherthan in the sediment without clams. All of DOC from subtidal sediment withclams was removed during biodegradability test, while in the sediment without clams; only about 60%of DOC was removed. Fig. 1 also shows that DOC concentration in subtidalsediment was lower than in tidal sediment and DOC concentration in subtidalsediment with clams was only about 42% of DOC concentration in the sedimentwithout clams. This result indicated that transfer rate of organic matter fromoverlying water to subtidal sediment was lower than that in tidal sediment.Immersion and emersion cycle in tidal sediment might be increased accumulationof organic matter in tidal sediment. This phenomenon also shown that clamsaccelerated organic matter degradation in subtidal sediment, but thecontribution of clams was relatively low.This result is consistent with the results of NH4N concentration in subtidal sediment, ashas been previously described.
3.2Changes in microbial community structure
The changes of chemicals environment in the sediment with clams, as has been described, promoted changes in microbial community structure, which indicated by changes in microbial quinone profile. Fig. 2 and 3 show that clam increase quinone content in tidal and subtidal sediments. Quinones exist in almost all
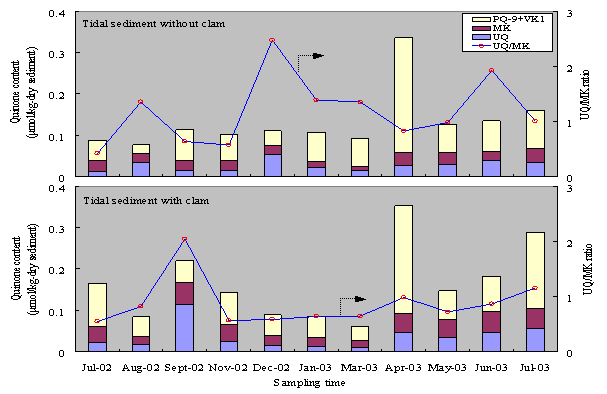
Figure 2. Monthly variations of quinone content in tidal flat sediment with and without clams
Figure 3. Monthly variations of quinone content in subtidal flat sediment with and without clams
microorganisms and play an important rolein the electron transport for respiration. In general, one species or genus of bacteria has only onedominant type of respiratory quinone.So, the quinone profile can be used as an index to characterize themicrobial community [17, 26, 27].Also, quinone content can be used as an index for the amount of biomass [17, 28]. Therefore, both microbial communitystructure and the concentration of biomass were simultaneously quantified byanalyzing quinones in a microbial community.
During July 2002 – July 2003, the averagequinone content in tidal sediment with clams was about 26% higher than in the sediment withoutclams. More detail, the concentrations of UQ, MK, and PQ+VK1 in tidal sediment with clamswere about 34, 55,and 15% higher than in the sediment without clams, respectively. This indicated thatthrough their excretion activity, clams improved the growth rates ofmicroorganism in tidal sediment,especially MK containing bacteria. High biodegradable compounds from clamexcretion activities are easier to consume by microorganism for growth and theirmetabolism. Clam activities and mineralization of biodegradable organic matterby microorganisms consumed DO and caused DO concentration in tidal sediment with clams was lower than in thesediment without clams. This condition was suitable for MK-containing bacteriagrowth, and causedUQ/MK ratio in the sediment with clams was lower than in the sediment withoutclams. UQ/MK ratios in thesediment with and without clams were 0.8 and 1.1, respectively. This indicated that clams changed thedominant group of bacteria and caused MK-containing bacteria become dominant intidal sediment with clams.
The effect of clams on changes in microbial communitystructure in subtidal sediment was relatively low. The averageof quinone content in tidal sediment with clams was only about 3% higher thanin the sediment without clams. In subtidal sediment, clams increased UQ containing bacteria growth. The existenceof clams increased pore size in the sediment and caused transport DO fromoverlying water to pore water become easier. Higher DO concentration in thesediment with clams promoted UQ containing bacteria growth and inhibited MKcontaining bacteria growth. The concentrations of UQ and PQ+VK1 in subtidalsediment with clams were about 33% and 6% higher than in the sediment withoutclams, respectively. While the concentration of MK in subtidal sediment with clams was about 26% lowerthan in the sediment without clam. These results indicated that clams have nosignificant effect to accelerate microbial growth in subtidal sediment. The changes of microbial communitystructure in subtidal sediment was not caused by clams activities, but might becaused by changes in physical properties of the sediment.
The fluctuation of organic matter andnutrient concentrations in seawater also affected the abundance anddensity of microorganism in the sediment. Nutrient concentration due to bivalve excretion wasalso fluctuated within a year [10]. It was observed thatquinone content,number of quinone species, and diversity of quinone were fluctuated within a year. Accumulation of organicmatter during summer season is usually followed by increase of abundance anddensity of microorganism at early autumn.
During one-year observation (July 2002 –July 2003), the diversity of quinone was fluctuated from 6.1 to 8.7 and from 5.0 to 8.8 in tidal sediment with andwithout clams, respectively. While in subtidal sediment,the diversity of quinone was fluctuated from 4.0 to 8.4 and from 4.5 to 8.7 inthe sediment with and without clams, respectively.Variations of seawater qualityand environmental condition influenced excretion activities of clams andbiodegradability of organic matter in the sediment. Finally, the microbialcommunity structure in the sediment was affected by these variations. Fig. 4 shows that clams decreased fluctuations ofdiversity and fraction dominant quinone. This indicated that clams improved thestability of microbial community structure in tidal and subtidal sediment overa year. Clams also tend to increasethe diversity of quinone especially in tidal sediment. These results indicatedthat the effect of clam on microbial community structure in tidal sediment wasmore significant than in subtidal sediment.
According to heterotrophic bacteria, the diversity of quinone was calculated from the composition of respiratory quinones (including UQ and MK). Fig. 5 shows that clams increased the diversity of respiratory quinones (DQuq+mk) in tidal sediment, but not in subtidal sediment. Fraction dominant quinone in tidal sediment with clams also more stable than in tidal sediment without clams or subtidal sediment. The diversities of respiratory quinones were fluctuated from 8.3 to 11.6 and 6.9 to 11.3 in tidal sediment with and without clams, respectively. While in subtidal sediment, the diversities were fluctuated from 8.0 to 10.4 and 9.4 to 12.1 in tidal sediment with and without clams, respectively. These results give more evident that clams affected microbial community structure especially heterotrophic bacteria in tidal sediment.
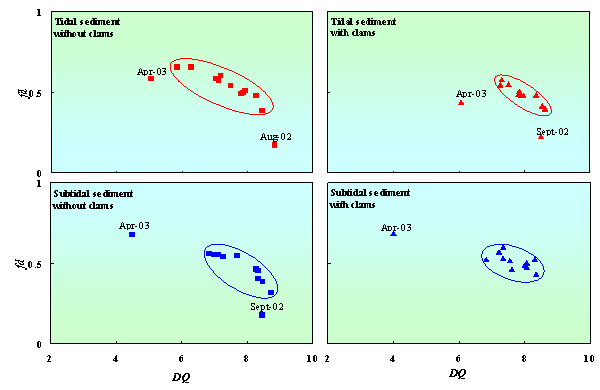
Figure 4. Changes in diversity of quinone (DQ) correspond to fraction dominant of quinone (fd) in tidal and subtidal sediment with and without clams.
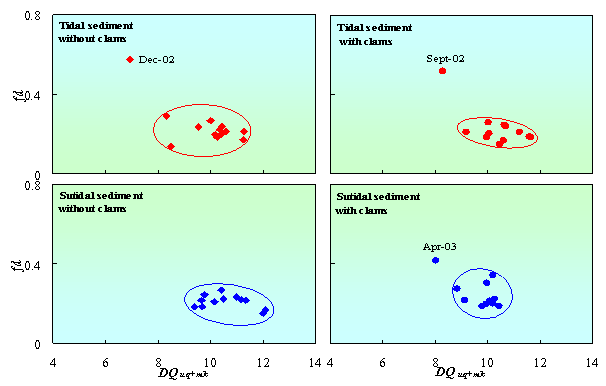
Figure 5. Changes in diversity of respiratory quinones (DQuq+mk) correspond to fraction dominant of quinone (fd) in tidal and subtidal sediment with and without clams.
Thedissimilarity between quinone profiles in the sediment with and without clams (Dwith, withoutclams)is shown in Fig. 6. Results show that the dissimilarity wasfluctuated within a yearobservation.Probably, it is dueto the fluctuation of organic matter concentration in seawater. The annualchlorophyll-a concentration in Mikawa Bay fluctuated from 83 to 149 mg chl-a·m–2for microphytobenthos (at 0–1 cm of the surface layer of sediments) and from0.5 to 15.3 mg chl-a·m–2 for phytoplankton (at a water depth of 1 min the intertidal flat) [29]. In enclosed coastal sea near largecity, such as Mikawa Bay, eutrophication of the water column occurs, and,consequently, there is excessive organic loading on the seabed, especiallyduring summer months [5]. Microorganism and clams (if any) will degradethese biomasses to produce higher biodegradable compoundsseveral times after. Therefore, during summer till beginning of autumn, theabundance and density of microorganism in the sediment with clams were higherthan that in the sediment without clams. This condition caused high level ofdissimilarity index between microbial community structure in tidal and subtidal sedimentwith and without clams. Othersenvironmental factor, such as temperature and DO are also influencing microbialcommunity structure in tidal flat sediment. It was observed that the highest effect of clams on themicrobial community structure occurred at the beginning of autumn (September2002). Moreover, in this time microbial concentration (quinone content) in thesediment with clams also much higher than in the sediment without clams. This strongly suggestedthat the utilization of clams on the improvement of tidal flat purificationcapacity is effective during summer till early autumn. D(with,without clams) in tidalsediment was higher than in subtidal sediment. From a year observation, the average of D(with,without clams) indices in tidaland subtidal sediment were 20% and 14%, respectively. Thepresence of clams increased DOC and NH4-N concentrations in tidaland subtidal sediment as described previously, causing changes in chemicalenvironments. Clamactivities also increased DO consumption rate in the sediments. These conditions mightlead to change in microbialcommunity structure in the sediments.
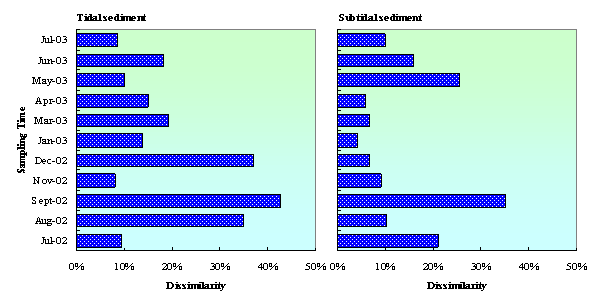
Figure 6. Dissimilarity between quinone profile in tidal and subtidal sediment with and without clams (Dwith, without clams)
3.3Tidal flat purification capacity
In this study, NO3-Nremoval capacity was used to evaluate the purification capacity of tidal flat sediment. Figure7 shows that high concentration of microorganism (quinone content) inthe sediment has correlation with high removal rate of NO3-N fromthe sediment, especially in the sediment with clams. The increases of carbonand nutrient concentration with higher biodegradability promoted microbialgrowth in the sediment with clams, which is indicated by higher concentration ofquinone. Quinone content in the sediment with clams (A) was higher than that inthe sediment without clams (B). Also, quinone content in 0-2 cm depth ofsediment was higher than that in 9-11 cm depth of sediment. This could berelated with
Figure 7. NO3-N removal rate in tidal flat sediment with (A) and without (B) clams. Index 1 and 2 indicated 0-2 cm and 9-11 cm depth of sediment, respectively
DO and substratelimitation in the deeper sediment. High concentration of microorganism in the sediment withclams was followed by higher NO3-N removal rate. While, in the caseof tidal sedimentwithout clams, higher concentration of microorganism in 0-2 cm depth of sedimentwas not followed by higher NO3-N removal rate proportionally. Thisindicated that microbial communities in the sediment with and without clams aredifferent. NH4-N concentration in tidal sediment with clams wasabout 3times higher than in the sediment without clams (Table 1). This conditionwas suitable for the growth of nitrifying bacteria and increase nitrificationprocess in tidal sediment with clams. Theavailability of NH4+ is one of major factors regulatingnitrification in the coastal marine sediment [30].Higheractivity of nitrifying bacteria produced higher concentration of NO3-N.Also, the existence of clams consumed DO and decreased DO concentration in thesediment. Low oxygen concentration or anaerobic condition is suitable for thegrowth of denitrifying bacteria [30]. NO3-N removal capacity in 2cm of the top layer of tidal flat sediment with clams was about 3 times higher than that of tidal flat sediment without clams. Highconcentration of denitrifying bacteria and low oxygen concentration weresuitable conditionsfor NO3-N removal from tidal flat sediment. High concentration of photosynthetic(photoheterotrophic) bacteria, which indicated by PQ and VK1, also increased NO3-Nremoval rate through NO3-N utilization as a terminal electronacceptor in their metabolism [31].The increased of sediment depthdecreased NO3-N removal capacity. This could be related with low concentration of microorganism andthe absence of photoheterotrophic bacteria in the lower sediment.
4.Conclusions
Clams appear to be a suitable organism to accelerate the decomposition of organic matter and nutrient removal in tidal flat sediment by improved biodegradability and promoted microbial growth in the sediment. This condition caused changes in microbial community in the sediment. The existence of clams increased quinone content, number of quinone species, and diversity of quinone in tidal sediment. Variations of seawater quality and environmental condition also influenced dissimilarity between microbial community structure in the sediment with and without clams. Changes in microbial community structure increased nitrate removal capacity in the sediment with clams. Results of this study are useful as additional information to improve tidal flat purification function and prevent eutrophication in the coastal area.
Acknowledgements
The authors thank Mr. KazuyaTakeda and Mr. Kazuhiro Shimada for their technical This research was supportedfinancially by the 21st Century COE (Center of Excellence) Project, 乬EcologicalEngineering for Homeostatic Human Activities乭 funded by the Ministry ofEducation, Culture, Sport, Science and Technology, Japan. References
[1] Zhao, B. Kreuter, U., Li, B., Ma, Z., Chen, J. and Nakagoshi, N.: An ecosystem service assessment of land-use change on Chongming Island, China. Land Use Policy, 21, 139-148. 2004.
[2] Ogilvie, B., Nedwell, D.B., Harrison, R.M., Robinson, A. and Sage, A.: High nitrate, muddy estuaries as nitrogen sinks: the nitrogen budget of the River Colne estuary (United Kingdom). Mar. Ecol. Prog. Ser., 150, 217-228. 1997.
[3] Magalhaes, M. C., Bordalo, A. A. and Wiebe, J.W.: Intertidal biofilms on rocky substratum can play a major role in estuarine carbon and nutrient dynamics. Mar. Ecol. Prog. Ser., 258, 275-281. 2003.
[4] Meyer-Reil, L.A. and Koster M.: Eutrophication of Marine Waters: Effects on Benthic Microbial Communities. Mar. Poll. Bull., 41, 255-263. 2000.
[5] Ueda, N., Tsutsumi, H., Yamada, M. and Hanamoto, K.: Impacts of oxygen-deficient water on the macrobenthic fauna of Dokai Bay and on adjacent intertidal flats, in Kitakyushu, Japan. Mar. Poll. Bull., 40, 906-913. 2000.
[6] Okabe, S., Matsuda, T., Satoh, H., Itoh, T. and Watanabe, Y.: Sulfate reduction and sulfide oxidation in aerobic mixed population biofilms. Wat. Sci. Technol., 37(4-5), 131-138. 1998.
[7] Graco, M., Farias, L., Molina, V., Gutierrez, D. and Nielsen, P.L.: Massive developments of microbial mats following phytoplankton blooms in a naturally eutrophic bay: implication for nitrogen cycling. Limnol. Oceanogr., 46 (4), 821-832. 2001.
[8] King, M. G.: Characterization of b-glucosidase activity in intertidal marine sediment. Appl. Env. Microbiol., 51, 373-380. 1986.
[9] Rutters, H., Sass, H., Cypioka, H. and Rullkotter, J.: Microbial communities in Wadden Sea sediment core-clues from analyses of intact glyceride lipids, and released fatty acids. Org. Geochem., 33, 803-816. 2002.
[10] Magni, P., Montani, S.,Takada, C. and Tsutsumi, H.: Temporalscaling and relevance of bivalve nutrient excretion on a tidal flat of the SetoInland Sea, Japan. Mar. Ecol. Prog. Ser., 198, 139-155. 2000.
[11] Hata,K., Nakata, K. and Suzuki, T.: The nitrogen cycle in tidal flats and eelgrassbeds of Ise Bay. J. Mar. Systems, 45, 237-253. 2004.
[12] Pfeiffer, J. T., Lawson,B. T. and Rusch, A. K.: Northern quahog,Mercenaria mercenaria, seed clam waste characterization study: precursorto a recirculating culture system design. Aquacul. Eng., 20, 149-161. 1999.
[13] Zhu, S.,Saucier, B., Durfey, J., Chen, S. and Dewey, B.:Waste excretion characteristics of Manila clams (Tapes philippinarum)under different temperature conditions. Aquacul. Eng., 20, 231-244.1999.
[14] Hasanudin,U., Shimada, K., Kunihiro, T., Fujie, K. and Suzuki, T.:The effect of clams on carbon and nitrate removal potential of tidal flat sediment.(Proceeding). Japan Society on Water Environment (JSWE)Annual Conference, March 17-19, Sapporo,Japan.2004.
[15] Pitter, P.and Chudoba, J.: Biodegradability oforganic substances in the aquatic environment. CRC Press, Boca Raton,Florida, USA. pp. 143-158. 1990.
[16] Hu, H. Y.,Fujie, K. and Urano, K.: Developmentof a novel solid phase extraction method for analysis of bacteria quinones inactivated sludge with higher reliability. J. Biosci. Bioeng., 87, 378-382. 1999.
[17] Hu, H. Y.,Lim, B.R., Goto, N. and Fujie, K.: Analyticalprecision and repeatability of respiratory quinones for quantitative study ofmicrobial community structure in environmental samples. J. Microbiol.Methods, 47, 17-24. 2001.
[18] Hiraishi, A.,Morishima, Y. and Takeuchi, J.: Numericalanalysis of lipoquinone pattern in monitoring bacterial in wastewater treatmentsystems. J. Gen. Appl. Microbiol., 37, 57-70. 1991.
[19] Tamaoka, J.,Katayama-Fujimura, Y. and Kuraishi H.:Analysis of bacterial menaquinone mixture by high performance chromatography.J. Appl Bacteriol., 54: 31-36. 1983.
[20] Kroger A.:Determination of contents and redox states of ubiquinone and menaquinone.Methods Enzymol., 53: 579-591. 1978.
[21] Sempere, R.,Yoro, C. S., Wambeke, F. V. and Charriere, B.:Microbial decomposition of large organic particles in the northwesternMediterranean Sea: an experimental approach. Mar. Ecol. Pro. Ser., 198, 61-72. 2000.
[22]; Durand, F.Peters, L.D. and Livingstone, D.R.: Effect ofintertidal compared to subtidal exposure on the uptake, loss, and oxidativetoxicity of water born benzo[a] pyrene in themantle and whole tissues of mussel Mytilus edulis, L. Mar. Env. Res., 54,271-274. 2002.
[23] Kuwae, T.,Kibe, E. and Nakamura, Y.: Effect ofemersion and immersion on the porewater nutrient dynamics of an intertidalsandflat in Tokyo Bay. Est. Coast. Shelf Sci., 57, 929-940. 2003.
[24] Karner, M.and Herndl G. J.: Extracellular enzymeactivity and secondary production in free living and marine-snow-associated bacteria.Mar. Biol., 113, 341-347. 1992.
[25] Smith, D.C.,Simon, M., Alldredge, A. and Azam, F.: Intensehydrolytic enzyme activity on marine aggregates and implications for rapidparticles dissolution. Nature, 359, 139-142. 1992.
[26] Hiraishi, A.: Isoprenoid quinones as biomarkers of microbial populations in theenvironment. J. Biosci.Bioeng., 88(5), 449-460. 1999.
[27] Hu, H. Y., Nozawa, M., Fujie, K., Makabe, T. and Urano, K.:Studies of microbial acclimation to hard chemicals on the basis of respiratoryquinone profiles and kinetic analyses. Wat. Sci. Technol., 34(5-6),249-256. 1996.
[28] Hiraishi, A., Ueda, Y. and Ishihara, J.: Quinoneprofiling of bacterial communities in natural and synthetic sewage activatedsludge for enhanced phosphate removal.Appl. Env. Microbiol., 64, 992-998. 1998.
[29] Goto, N., Mitamura, O. and Terai, H.: Seasonalvariation in primary production of microphytobenthos at the Isshiki intertidalflat in Mikawa Bay. Limnol., 1, 133-138. 2000.
[30] Usui, T., Koike, I. and Ogura, N.: N2Oproduction, nitrification and denitrification in an estuarine sediment. Est.Coast. Shelf Sci., 52, 769-781. 2001.
[31] Lester, J.N. and Birkett, J.W.: Microbiology andchemistry for environmental scientists and engineers. (2nd edition).E & FN Spon, London. pp. 325-337. 1999.
论文搜索
月热点论文
论文投稿
很多时候您的文章总是无缘变成铅字。研究做到关键时,试验有了起色时,是不是想和同行探讨一下,工作中有了心得,您是不是很想与人分享,那么不要只是默默工作了,写下来吧!投稿时,请以附件形式发至 [email protected] ,请注明论文投稿。一旦采用,我们会为您增加100枚金币。








